UTILITIES
May 19, 2020
We are truly living in unique times. The coronavirus pandemic is impacting everyone from our families, to our businesses, to our first responders who are bravely on the front lines. As the situation continues to affect our world, ITA GROUP realized a preventive procedure to support food industries, retail, employees, our community and you—our customers and partners.
May 9, 2017
The U.S. Food and Drug Administration took a step towards ensuring the accuracy of its inventory of importers responsible for meeting the requirements of the Foreign Supplier Verification Programs (FSVP) rule.
May 8, 2017
Establishing risk-based preventive controls enables you to apply a proactive and systematic approach to your food safety program through the establishment of preventive controls designed to protect your food products, and the consumer, from biological, chemical (including radiological), and physical hazards.
May 7, 2017
The regulatory framework to determine if a business falls under the definition of “farm” depends mostly on certain denotations (i.e. “farm,” “mixed-type facility", including a “farm mixed-type facility”, “harvesting,” “packing,” “packaging,” “holding” and “manufacturing/processing”) which are identified within the regulation for Registration of Food Facilities (21 CFR part 1, subpart H)[see note].
May 6, 2017
This guidance is intended to assist industry and the Food and Drug Administration (FDA) staff by recommending standards for accrediting third-party certification bodies for the voluntary third-party certification program established under the FDA Food Safety Modernization Act (FSMA). The guidance serves as a companion document to the implementing regulations in 21 CFR parts 1, 11, and 16 that establish the framework, procedures, and requirements for accreditation bodies and third-party certification bodies for this program.
May 5, 2017
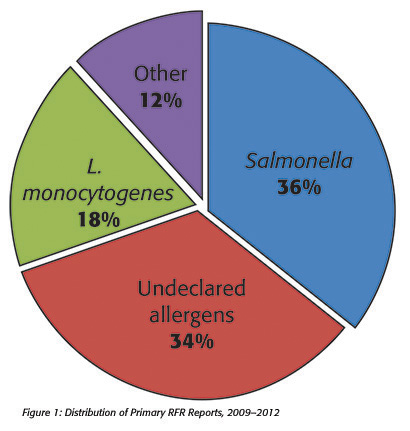
The Reportable Food Registry (RFR or the Registry) was established by Section 1005 of the Food and Drug Administration Amendments Act of 2007 (Pub. L. 110-85), which amended the Food, Drug, and Cosmetic Act (FD&C Act) by creating a new Section 417, Reportable Food Registry [21 U.S.C. 350f].
May 4, 2017
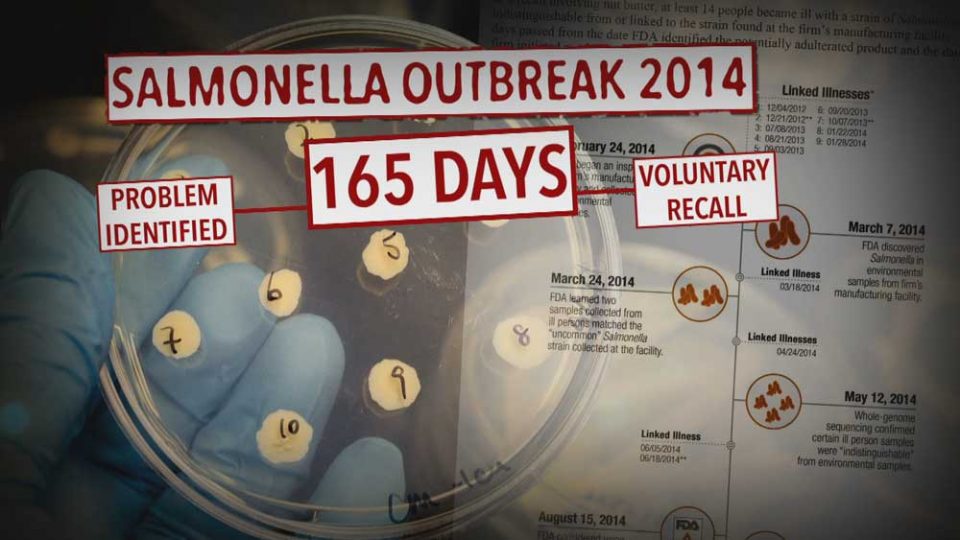
All recalls monitored by FDA are included in the Enforcement Report once they are classified. Information about how to navigate the report and for definitions of the report labels are found on the Enforcement Report Navigation and Definitions page.
May 3, 2017
If your product is detained without physical examination, you have the right to provide evidence to FDA in an attempt to overcome the appearance of the violation. If you do not provide evidence to FDA, or if the information you provide is not sufficient to overcome the appearance of the violation, your product is subject to refusal into the United States. Visit the Detention and Hearing page for more information on this process. Contact the compliance officer listed on your FDA Notice of Action if you have questions related to the detention.
May 2, 2017
The list below provides information gathered from press releases and other public notices about certain recalls of FDA-regulated products.
Not all recalls have press releases or are posted on this page. See Additional
information about recalls for a more complete listing.