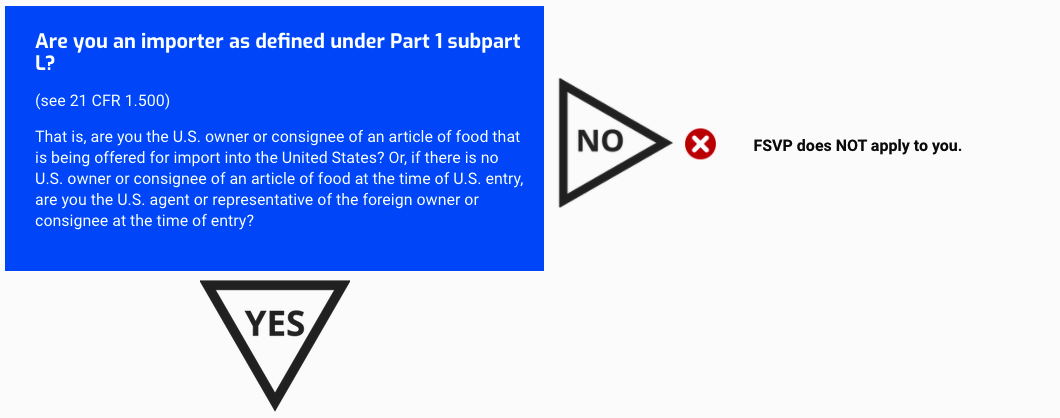
Are you an importer as defined under Part 1 subpart L?
(see 21 CFR 1.500)
That is, are you the U.S. owner or consignee of an article of food that is being offered for import into the United States? Or, if there is no U.S. owner or consignee of an article of food at the time of U.S. entry, are you the U.S. agent or representative of the foreign owner or consignee at the time of entry?



FSVP does NOT apply to you.
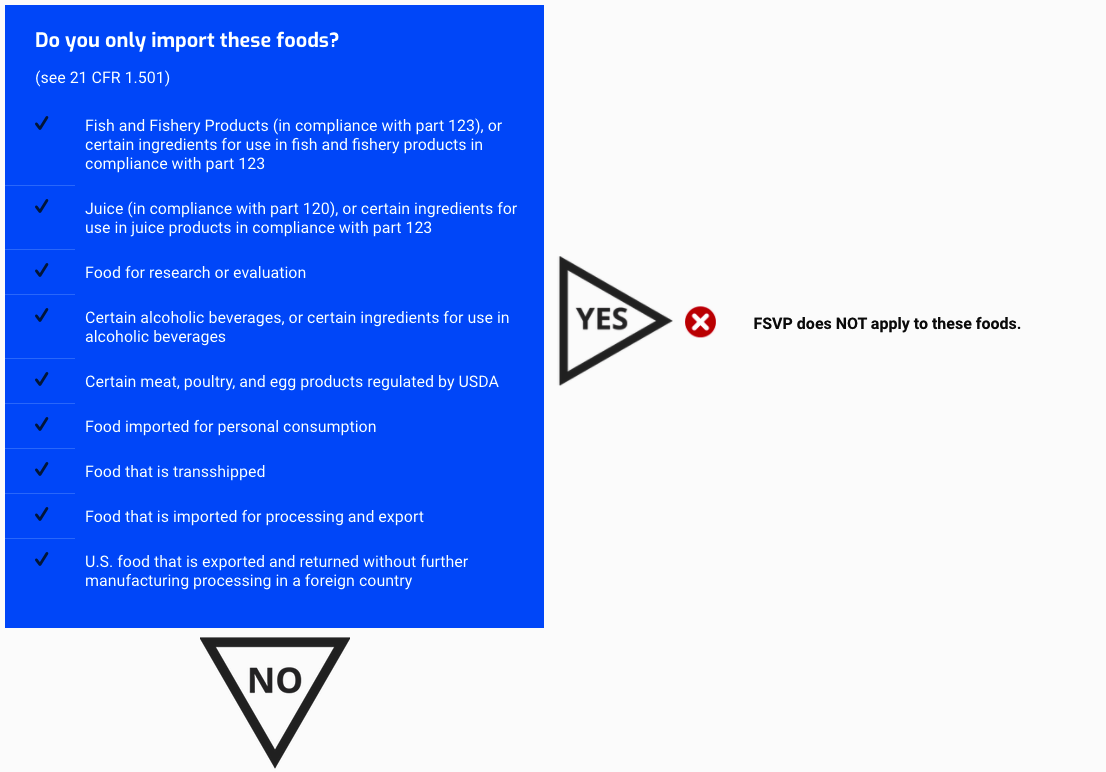
Do you only import these foods?
(see 21 CFR 1.501)
- Fish and Fishery Products (in compliance with part 123), or certain ingredients for use in fish and fishery products in compliance with part 123
- Juice (in compliance with part 120), or certain ingredients for use in juice products in compliance with part 123
- Food for research or evaluation
- Certain alcoholic beverages, or certain ingredients for use in alcoholic beverages
- Certain meat, poultry, and egg products regulated by USDA
- Food imported for personal consumption
- Food that is transshipped
- Food that is imported for processing and export
- U.S. food that is exported and returned without further manufacturing processing in a foreign country



FSVP does NOT apply to these foods.
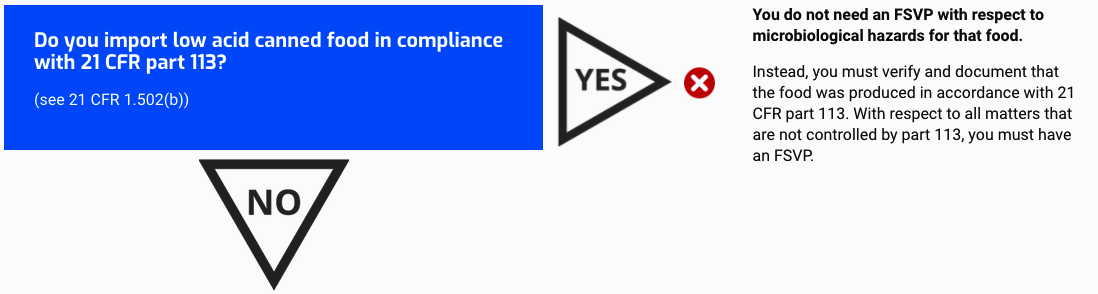
Do you import low acid canned food in compliance with 21 CFR part 113?
(see 21 CFR 1.502(b))



You do not need an FSVP with respect to microbiological hazards for that food.
Instead, you must verify and document that the food was produced in accordance with 21 CFR part 113. With respect to all matters that are not controlled by part 113, you must have an FSVP.
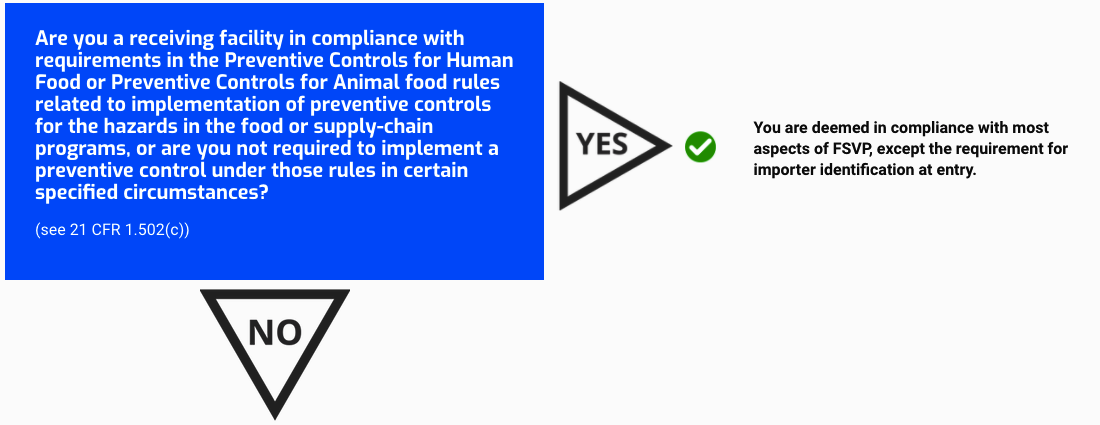
Are you a receiving facility in compliance with requirements in the Preventive Controls for Human Food or Preventive Controls for Animal food rules related to implementation of preventive controls for the hazards in the food or supply-chain programs, or are you not required to implement a preventive control under those rules in certain specified circumstances?
(see 21 CFR 1.502(c))



You are deemed in compliance with most aspects of FSVP, except the requirement for importer identification at entry.
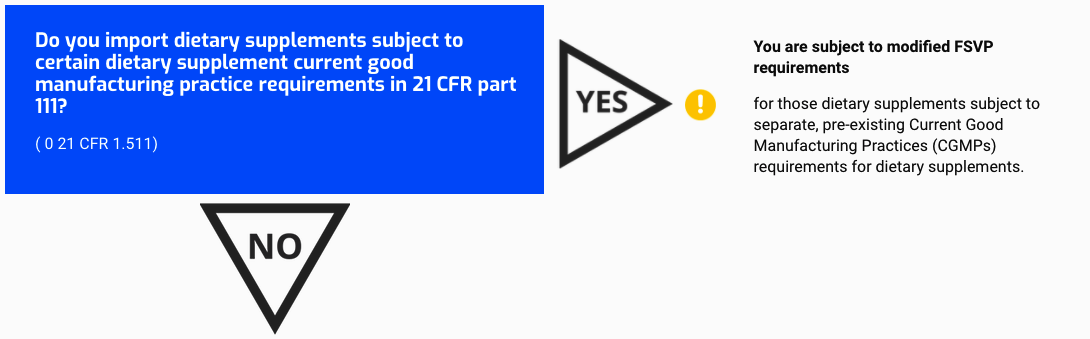
Do you import dietary supplements subject to certain dietary supplement current good manufacturing practice requirements in 21 CFR part 111?
(0 21 CFR 1.511)



You are subject to modified FSVP requirements
for those dietary supplements subject to separate, pre-existing Current Good Manufacturing Practices (CGMPs) requirements for dietary supplements.
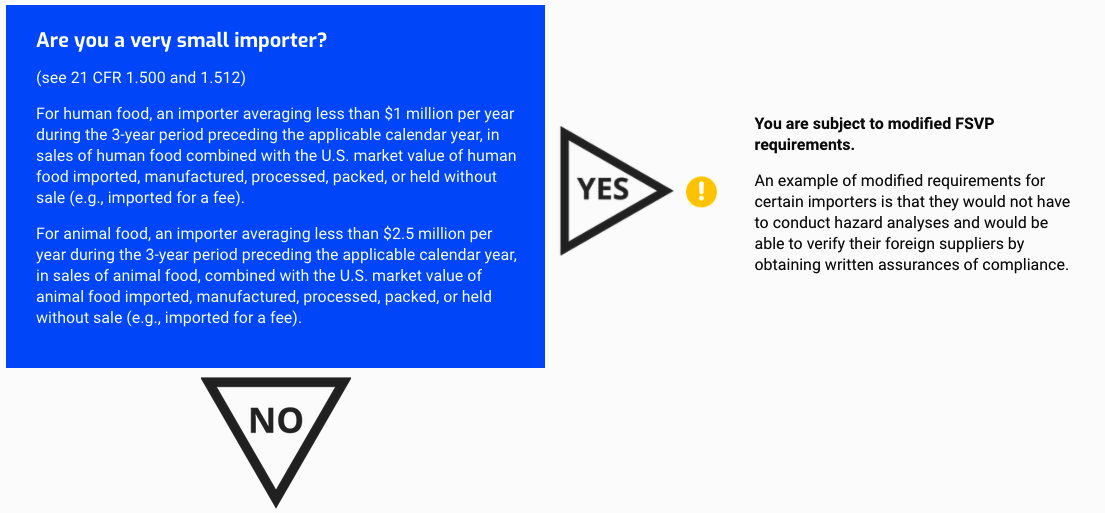
Are you a very small importer?
(see 21 CFR 1.500 and 1.512)
For human food, an importer averaging less than $1 million per year during the 3-year period preceding the applicable calendar year, in sales of human food combined with the U.S. market value of human food imported, manufactured, processed, packed, or held without sale (e.g., imported for a fee).
For animal food, an importer averaging less than $2.5 million per year during the 3-year period preceding the applicable calendar year, in sales of animal food, combined with the U.S. market value of animal food imported, manufactured, processed, packed, or held without sale (e.g., imported for a fee).



You are subject to modified FSVP requirements.
An example of modified requirements for certain importers is that they would not have to conduct hazard analyses and would be able to verify their foreign suppliers by obtaining written assurances of compliance.
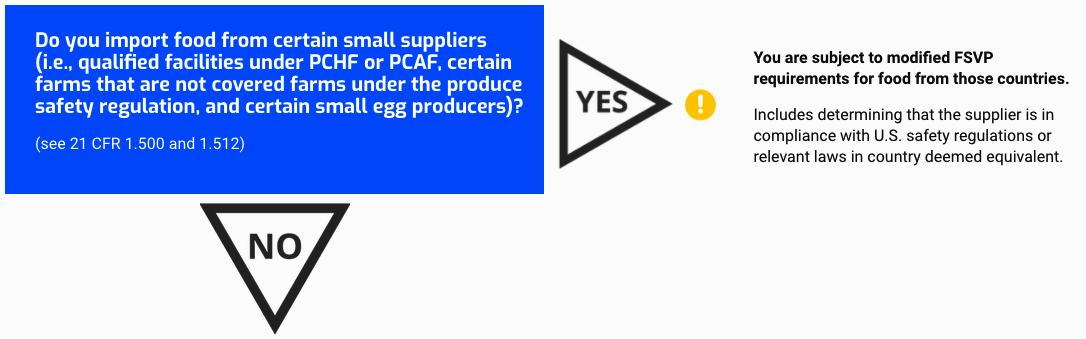
Do you import food from certain small suppliers (i.e., qualified facilities under PCHF or PCAF, certain farms that are not covered farms under the produce safety regulation, and certain small egg producers)?
(see 21 CFR 1.500 and 1.512)



You are subject to modified FSVP requirements for food from those countries.
Includes determining that the supplier is in compliance with U.S. safety regulations or relevant laws in country deemed equivalent.
YOU ARE SUBJECT TO FSVP.